Diagnostic Solutions
Research & Development
Diagnostic Solutions
Towards early Detection
‘Early detection is critical for early response’
Early detection and differential diagnosis of pathogens or the infection they produced increases the chances for successful control of any disease. Differential diagnosis must be based on laboratory testing, and its proper application is the foundation of disease prevention, control, and elimination programs.
Diagnostic Solutions
Disease Transitions Stages
Classically, the “natural history” of an infectious disease describes the “disease transition stage” through which an animal (or individual) passes over the course of the infection (Thurmond, 2003). Likewise, “diagnostic transition stage” describes the changes in diagnostic sensitivity and specificity that occur for specific combinations of specimen and test as animals passed through disease transition stages. Because of the dynamics of the infection, the probability of detecting any infection changes over time as a function of specimen and diagnostic target. Therefore, in general, effective pathogen detection is heavily dependent on the combination of specimens (serum, oral fluid, feces, mammary secretions, environmental samples) and direct (e.g., PCR) and indirect (e.g., ELISA) diagnostic tools.
Innoceleris is specialized in the ready-to-use reagents and assay platforms for animal infectious diseases, with particular emphasis on immunoassays for detection of pathogen-specific antibody (e.g., ELISA, immunofluorescence, Luminex, AlphaLISA).
Immunoassay Solutions for Swine Diseases
Innoceleris provide immunoassay expertise for pathogen-specific antibody (IgG, IgA and IgM) detection in different specimens including serum/plasma, oral fluid, processing fluid, mammary secretions, feces, etc.

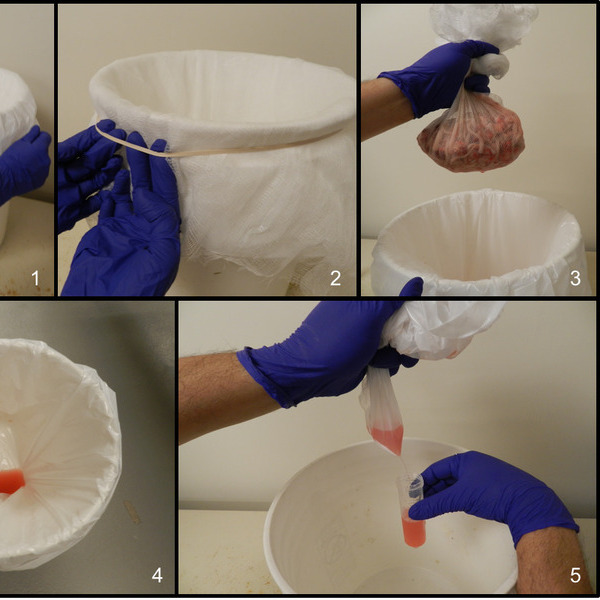
Diagnostic solutions for alternative specimens
Historically, serum has been the standard test specimen in diagnostic and surveillance. However, recent research explored non-invasive diagnostic alternatives to individual animal sampling and the potential for more efficient, responsive, and cost-effective surveillance. Indeed antibody are detectable in different body secretions and specimens other than serum, including buccal (e.g., oral fluids) and nasal secretions, respiratory exhalations (aerosols), mammary secretions (colostrum/milk), processing fluids, urine, fecal specimens, and environmental samples.
While most of the commercial immunoassays (mainly ELISAs) currently available are specifically design for serum/plasma samples, Innoceleris can adapt and/or develop different immunoassay platforms for various specimen types. Our work has been particularly focused on the most economically significant pathogens of production animals, e.g., African swine fever virus (ASFV), foot and mouth disease virus (FMDV), classical swine fever virus (CSFV), and porcine epidemic diarrhea virus (PEDV).
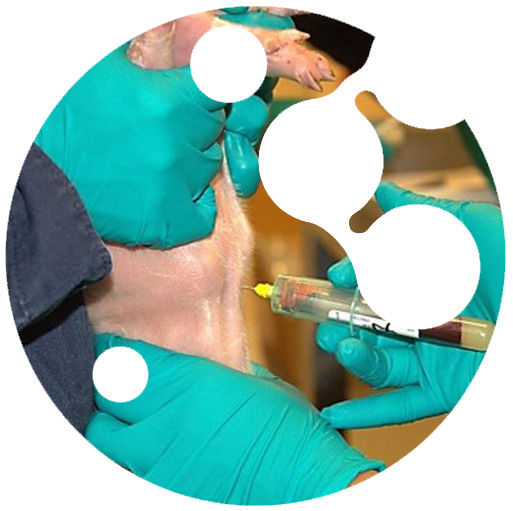
Validations Diagnostic Performance
“In God we trust; all others bring data…” (W. Edwards Deming)
The bigger obstacle for an accurate assessment of the diagnostic performance (diagnostic sensitivity and specificity) of any diagnostic tool is the lack of diagnostic specimens of precisely known infectious status. This is why all the diagnostic technology developed and validated by Innoceleris relies on the use of large panels of specimens of precisely known infectious status.